
PTAB Finds That Chemokine Receptor-Derived (CKRD) Peptides are Not Natural Products
The Patent Trial and Appeal Board (PTAB) reversed the § 101 rejection of claims directed to chemokine receptor-derived (CKRD) peptides, and found the claims to be eligible for patenting.1 This decision provides helpful insights on how the U.S. Patent and Trademark Office evaluates claims involving peptide fragments derived from naturally occurring proteins under 35 U.S.C § 101.
The Technology
The case involved Protagonists Ltd.’s peptides that were based on the nine amino acid sequence of Trp, Val, Phe, Gly, Ser, Gly, Leu, Cys, Lys. This peptide sequence is derived from a naturally occurring protein, namely a chemokine receptor protein.
As shown in the illustration below, chemokines bind to chemokine receptors, and thereby initiate inflammation as part of an effective immune response. In some cases, however, dysregulation of the chemokine receptor protein signaling can lead to autoimmune disorders or inflammatory diseases such as arthritis.
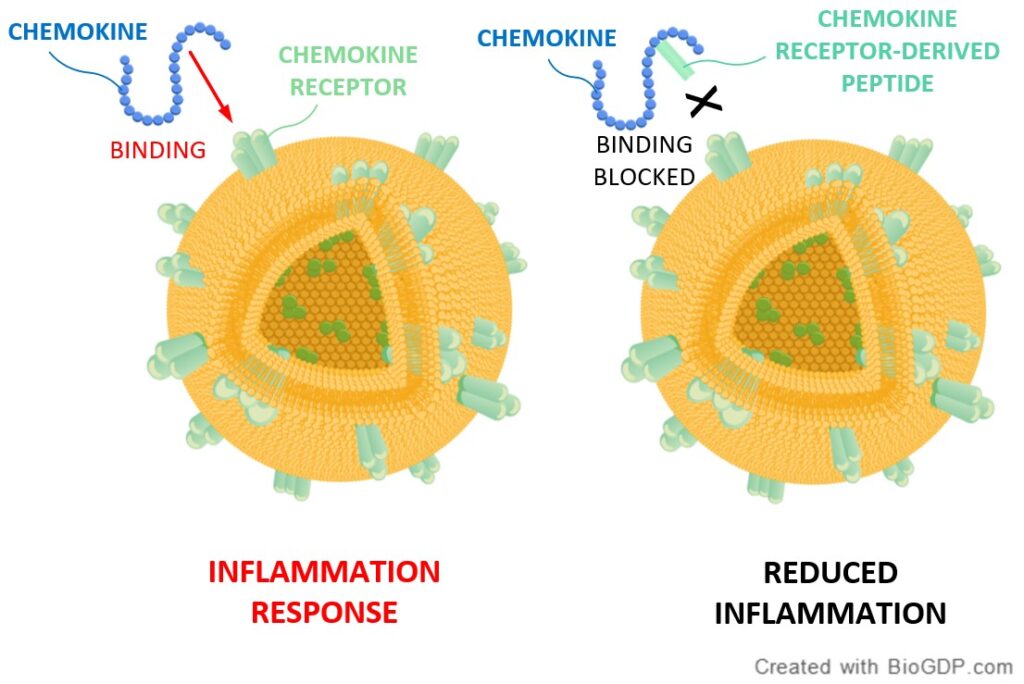
Here, the inventors discovered it was possible to use a specific portion of the chemokine receptor (just the part that usually binds to the chemokine) as a decoy, so that the chemokine would bind to this peptide segment instead of to the receptor itself. As a result, the chemokine receptor is not activated and the inflammation response is reduced.
The § 101 Rejection
The examiner rejected the claims under 35 U.S.C. §101, arguing they recited a natural phenomenon (e.g., product of nature). With regard to Step 1 (see flowchart below), the examiner found the claims to be directed to compositions of matter, namely peptides.
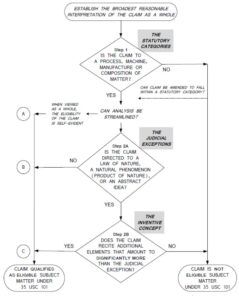
With regard to Step 2A Prong One (see flowchart below), the § 101 rejection concluded that the claims recited a natural phenomenon, because the peptide corresponded to domains of naturally occurring proteins which are a natural phenomenon. With regard to Step 2A Prong 2, the § 101 rejection concluded that the claims did not recite additional elements that integrated the judicial exception into a practical application.
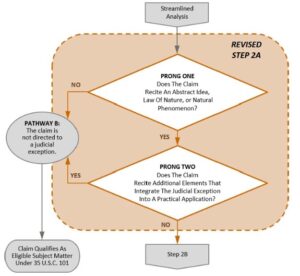
With regard to Step 2B, the § 101 rejection concluded that the claims did not recite additional elements that were sufficient to amount to significantly more than the judicial exception.
The Board’s Analysis
The PTAB reversed, disagreeing with various aspects of the § 101 rejection.
- Peptide Fragment is Not Product of Nature: The Board keyed in on the question of whether a peptide that is a fragment of a larger naturally occurring protein is inherently also naturally occurring. In this instance, the Board noted that the § 101 rejection provided no evidence that the claimed peptides were found in nature, even though it was theoretically conceivable that such a peptide may have previously naturally formed as a product of protease cleavage within the human body. According to the Board, “it is the Examiner’s burden to show that claimed subject matter is necessarily naturally occurring and not merely an unusual and rare phenomenon.”
- Markedly Different Characteristics: The Board found that even if the peptide could be considered as a nature-based product, it had markedly different characteristics than the naturally occurring protein based upon multiple lines of evidence, including binding activity, structure, and effect on inflammation.
In sum, the Board resolved the patent eligibility question under Step 2A Prong 1 by finding that the claimed peptide was not directed to a product of nature, without needing to complete an analysis that fully addressed Step 2A Prong 2 or Step 2B.
Key Takeaways for Patent Attorneys and Agents
This decision highlights important principles for patent practitioners dealing with compositions involving peptide fragments of larger naturally occurring proteins.
- Emphasize experimental evidence showing structural and functional differences from the natural protein source.
- Emphasize experimental evidence showing therapeutic properties of claimed peptides.
PERA Implications
Although the Board found that the claims met the requirements of § 101, under the currently proposed language of the Patent Eligibility Restoration Act (PERA, Senate Bill 2140) it is likely that this case would not have made it to the Board, as the examiner would likely have concluded that the composition claims met the patent eligibility requirement (e.g. pursuant to §§101(a), (b)(2)(A),(b)(2)(B)).
Related Reading
As noted above, the Board placed the burden on the Examiner to show that the claimed subject matter is necessarily naturally occurring and not merely an unusual and rare phenomenon (as part of the Step 2A Prong One analysis). Complement this result with the Board’s position in Ex parte Ma, where the Board seemed to implicitly put the burden of proof on the examiner to show that the claimed formulation lacked markedly different characteristics as compared to a product of nature (also as part of the Step 2A Prong One analysis).
1 Ex parte Ezerzer(Appeal 2022-004253; February 7, 2024)